When a patient is switched from a brand-name drug to a generic version, most people assume it’s just a cost-saving change. But for drugs with a narrow therapeutic index (NTI), that assumption can be dangerous. These aren’t ordinary medications. A tiny change in dose - even as small as 5% - can mean the difference between treatment working and something going seriously wrong. Think seizures returning, blood clots forming, or heart rhythm problems. That’s why how you talk to patients about switching NTI generics isn’t just important - it’s critical.
What Makes NTI Drugs Different?
NTI drugs have a very small window between the dose that works and the dose that harms. It’s not like taking an antibiotic, where you can miss a pill and still be okay. With NTI drugs, if your blood level drops too low, the treatment fails. If it rises too high, you risk toxicity. There’s no room for error. Examples include:- Warfarin - used to prevent blood clots. The target INR is 2-3. Too low? Clot risk. Too high? Bleeding risk.
- Phenytoin - an anti-seizure drug. Levels below 10 mcg/mL may not control seizures. Above 20 mcg/mL? Toxicity, including dizziness, nausea, and even coma.
- Levothyroxine - for thyroid replacement. Even small changes can cause fatigue, weight shifts, or heart palpitations.
- Digoxin - for heart failure. Therapeutic range is 0.5-0.9 ng/mL. A slight increase can cause life-threatening arrhythmias.
- Valproic acid and carbamazepine - both used for epilepsy and mood disorders. Small fluctuations can trigger seizures or mood crashes.
The FDA requires stricter testing for these generics. While regular generics must match the brand within 80-125% of the original drug’s absorption, NTI generics must stay within 90.00%-111.11%. That’s a tighter range. But even with this, confusion lingers.
Why Do Patients and Providers Still Worry?
A 2017 survey of pharmacists showed that 87% believed generic NTI drugs were just as effective. Yet only 60% consistently substituted them. Why? Because patients and even some doctors remember stories - a seizure returned after switching, an INR spiked, someone had to go to the ER. The truth? Most of these events aren’t caused by the generic itself. Studies show that when patients are properly monitored after a switch, outcomes are nearly identical to staying on the brand. But the fear sticks. And that’s where communication breaks down. Many providers say: “It’s the same drug.” But that’s not enough. Patients aren’t just asking about chemistry. They’re asking: “Will this hurt me? Will I get sick? Will I end up back in the hospital?”What You Must Say - And How to Say It
Don’t just inform. Educate. And do it with trust, not jargon. Here’s what works:- Start with empathy: “I know you’ve been stable on your current medication. Switching anything can feel risky - and you’re right to be cautious.”
- Use clear comparisons: “This generic has the exact same active ingredient. It’s not a different medicine. It’s been tested to release the same amount of drug into your bloodstream as the brand - within 90% to 111%.”
- Be honest about monitoring: “Because this is an NTI drug, we’ll check your blood levels in 5-7 days. That’s not because we think it won’t work - it’s because we want to make sure it works perfectly for you.”
- Lead with authority: “I prescribe this same generic to my own family. I trust it. And I’ll be monitoring you closely.”
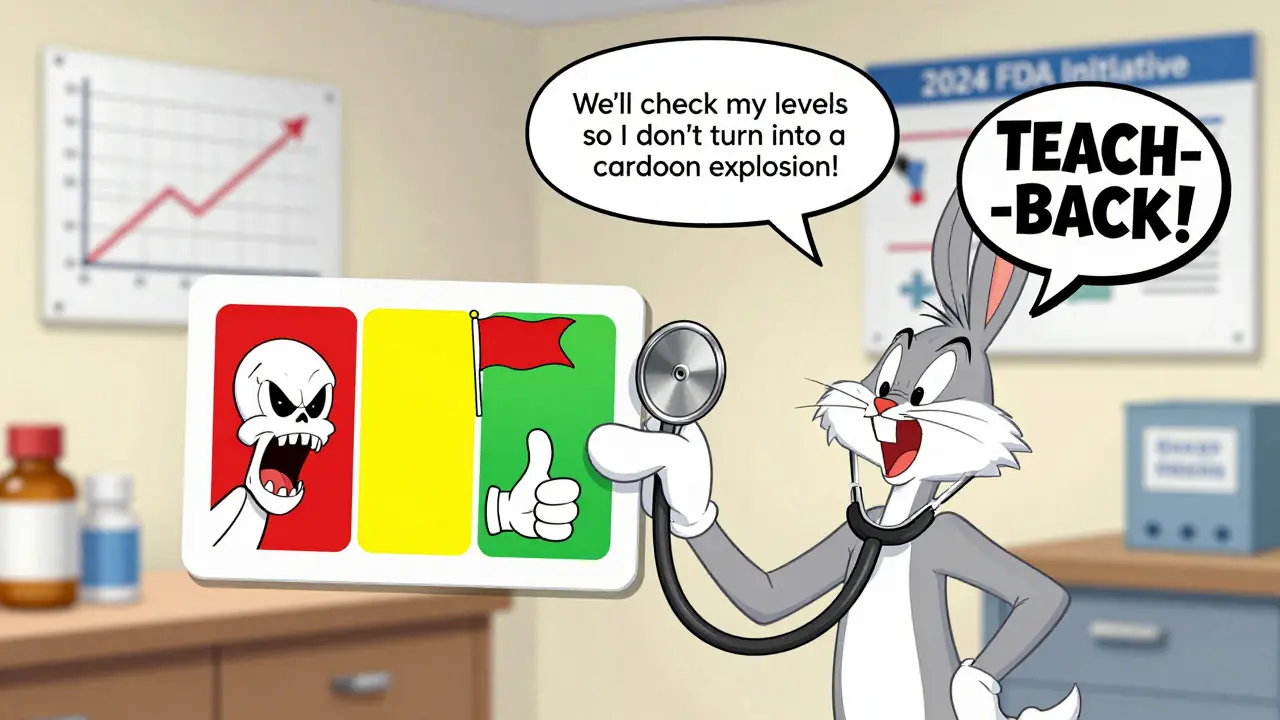
A 2020 study found that patients who received personalized counseling from pharmacists had 28% fewer medication-related problems. Visual aids helped too - showing a graph of the therapeutic window, or a simple chart comparing brand and generic absorption rates. One pharmacy in Birmingham started using a laminated card with a red zone (toxic), green zone (safe), and yellow zone (monitor). Patients kept it in their wallet. Adherence jumped 42%.

What to Check Before Switching
Before you even mention a switch, ask yourself:- Is this drug on the FDA’s NTI list? Check the official list - it includes warfarin, levothyroxine, phenytoin, digoxin, and others.
- What does your state require? In 27 U.S. states, pharmacists can’t switch NTI drugs without written patient consent. Fourteen states require you to get that consent before dispensing. In others, you can switch automatically - but you still need to counsel the patient.
- Who is the patient? Elderly patients? Those with kidney or liver issues? On multiple meds? These groups are more sensitive to changes. Monitor them extra closely.
- Is this the first switch? If the patient has switched before and had a problem, document it. Don’t assume the same outcome will happen again - but don’t ignore it either.
What to Document
Don’t rely on memory. Write it down - clearly and specifically. Use this template:- Patient counseled on therapeutic equivalence of generic [drug name] to brand version.
- Advised that blood monitoring (e.g., INR, serum level, TSH) will be performed within [X] days of switch.
- Written educational materials provided (FDA patient guide or pharmacy handout).
- Patient verbalized understanding using teach-back: “So you’ll check my blood in 5 days to make sure it’s still in the safe range?”
This isn’t just paperwork. It’s protection - for the patient and for you.
What Not to Say
Avoid these phrases - they sound dismissive:- “It’s the same thing.”
- “Don’t worry, it’s FDA-approved.”
- “The brand isn’t better.”
These shut down conversation. They don’t build trust. Instead:
- Use “we” language: “We’ll watch your levels together.”
- Use “you” language: “Your safety matters to us.”
- Use data: “Studies show that with proper monitoring, outcomes are the same.”
What’s Changing Now
In 2024, the FDA launched the NTI Drug Communication Initiative. It includes:- Standardized patient handouts in 12 languages.
- Counseling checklists for 15 high-risk NTI drugs.
- Recommendations for a minimum 10-minute discussion before any substitution.
- Use of teach-back: “Tell me in your own words what you’ll do after the switch.”
By 2025, the FDA plans to use real-world data from 12 million patients to track outcomes after NTI switches. This isn’t about proving generics are safe - we already know they are. It’s about proving we’re doing everything right when we switch them.
Bottom Line
Switching NTI generics isn’t a pharmacy decision. It’s a clinical one. And it demands more than a stamp on a prescription.Patients aren’t resisting generics because they’re irrational. They’re resisting because they’ve been given incomplete information. Your job isn’t to convince them. It’s to guide them - with clarity, with care, and with evidence.
When you explain why the switch is safe - and what you’ll do to protect them - they don’t just accept it. They trust it.
Are all generic drugs the same, even for NTI medications?
No. Generic drugs for narrow therapeutic index (NTI) medications must meet stricter standards than regular generics. While most generics must be within 80-125% of the brand’s absorption, NTI generics must stay within 90.00%-111.11%. This tighter range ensures blood levels stay within the safe and effective window. The FDA has specific guidelines for drugs like warfarin, levothyroxine, and phenytoin, requiring more precise testing before approval.
Can I switch my patient from brand to generic without monitoring?
No. Even though FDA-approved NTI generics are therapeutically equivalent, monitoring is required after any switch. For warfarin, check INR within 3-5 days. For phenytoin or levothyroxine, check serum levels or TSH within 7-10 days. This isn’t optional - it’s standard practice. Skipping monitoring increases the risk of undetected changes in drug levels that could lead to treatment failure or toxicity.
Why do some pharmacists refuse to substitute NTI generics?
Some pharmacists avoid substitution due to state laws requiring patient consent, fear of liability, or outdated beliefs. A 2017 survey found pharmacists with over 20 years of experience were 37% less likely to substitute NTI generics than newer pharmacists. This isn’t because generics are unsafe - it’s because communication gaps and inconsistent policies have created lingering doubt. The FDA and American Pharmacists Association now recommend standardized counseling to reduce these barriers.
What states have special rules for NTI drug substitution?
As of 2024, 27 U.S. states have specific laws about NTI drug substitution. Fourteen of them require written patient consent before switching. Others limit substitution to certain situations or require prescriber authorization. For example, in California and New York, pharmacists must notify the prescriber if they switch an NTI drug. Always check your state’s pharmacy board rules - they override federal guidelines.
What should I do if a patient refuses a generic NTI switch?
Respect their decision. But don’t leave it at that. Ask why. Is it fear? A bad past experience? A misunderstanding? Provide clear, written materials from the FDA or your pharmacy. Offer to call the prescriber together. If they still refuse, document the refusal and keep them on the brand. Never pressure. Your goal isn’t to save money - it’s to keep them safe and informed.
Do NTI generics cost less than brand-name drugs?
Yes - but not always as much as non-NTI generics. Because of stricter manufacturing and testing requirements, NTI generics cost 15-30% less than the brand, compared to 70-80% savings for regular generics. Still, that’s significant savings for patients on long-term therapy. For example, a month’s supply of brand-name warfarin might cost $40, while the generic is $12. That’s still a 70% drop - even if it’s not as deep as with other drugs.
How do I know if a drug is on the FDA’s NTI list?
The FDA publishes product-specific guidance documents for NTI drugs. As of 2024, 37 drugs are confirmed as NTI and require enhanced bioequivalence standards. Common ones include levothyroxine, warfarin, phenytoin, digoxin, valproic acid, and carbamazepine. You can find the full list on the FDA’s Office of Generic Drugs website. Many pharmacy systems now flag NTI drugs automatically - but always double-check if you’re unsure.